TM 5-811-7
CHAPTER 1
INTRODUCTION TO CATHODIC PROTECTION
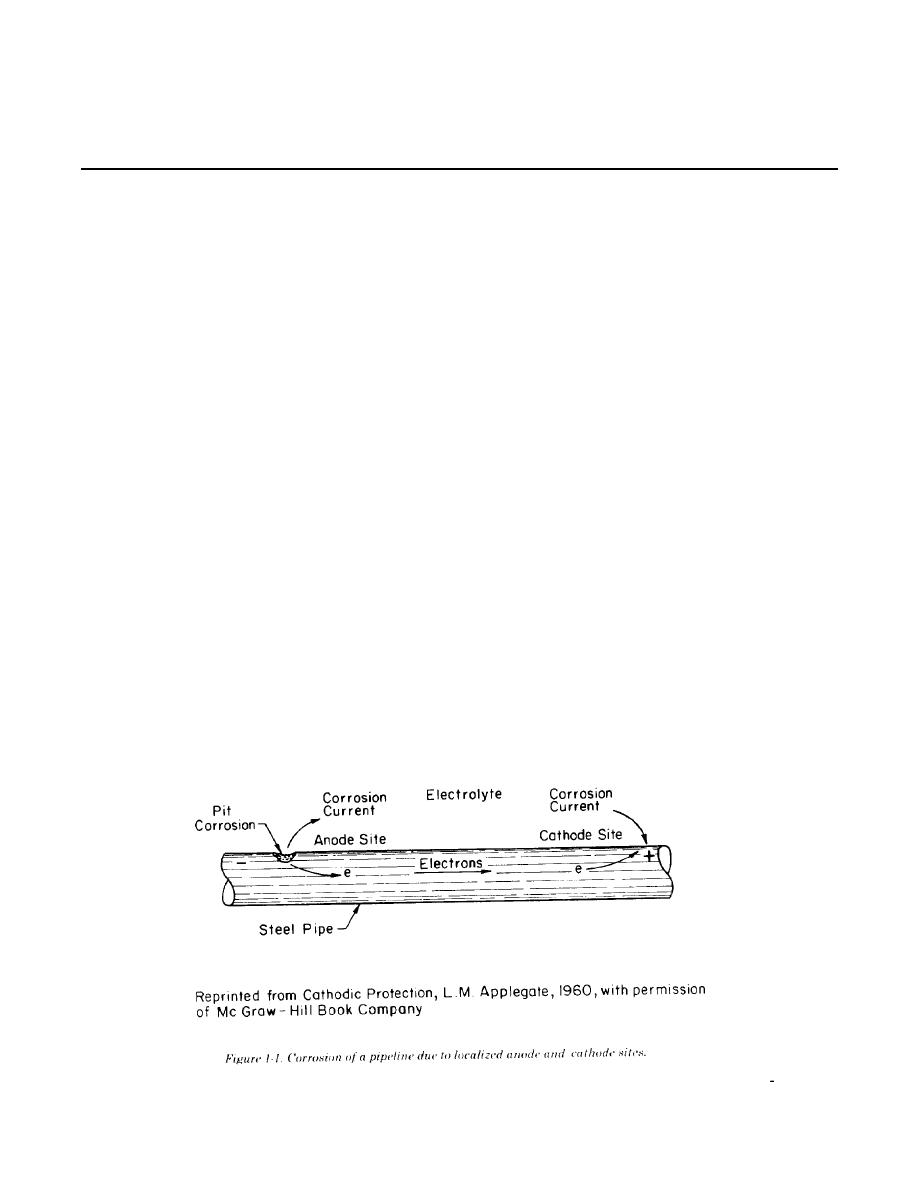
one small section of a pipeline may be anodic
1-1. Purpose.
because it is in a soil with low resistivity compared
This manual presents design guidance for cathodic
to the rest of the line. Current would leave the
protection systems.
pipeline at that anode site, pass through the soil,
1-2. References.
and reenter the pipeline at a cathode site. Current
a. Government publications.
flows because of a potential difference between the
Department of Transportation
anode and cathode. That is, the anode potential is
Superintendent of Documents, U.S. Gov-
more negative than the cathode potential, and this
ernment Printing Office, Washington, DC
difference is the driving force for the corrosion
20402
current. The total system--anode, cathode, electro-
Transportation of Natural and Other Gas by
lyte, and metallic connection between anode and
Pipeline: Minimum Federal Safety Stan-
cathode (the pipeline in fig 1-1)--is termed a
dards, Subpart 1 - Requirements Register,
corrosion cell.
Vol 36, No. 126 (June 30, 1971).
1-4. Cathodic protection.
b. Nongovernment publications.
Cathodic protection is a method to reduce corro-
National Association of Corrosion Engineers
sion by minimizing the difference in potential
(NACE), P.O. Box 218340, Houston, TX
between anode and cathode. This is achieved by
77084
applying a current to the structure to be protected
Standard RP-01-69 Control of External
(such as a pipeline) from some outside source.
(1972 revision
Corrosion on Under-
When enough current is applied, the whole struc-
ground or Submerged
ture will be at one potential; thus, anode and
Metallic Piping Systems
cathode sites will not exist. Cathodic protection is
Standard RP-02-72 Direct Calculation of
commonly used on many types of structures, such
Economic Appraisals
as pipelines, underground storage tanks, locks, and
of Corrosion Control
ship hulls.
Measures
1-5. Types of cathodic protection systems.
1-3. Corrosion.
There are two main types of cathodic protection
Corrosion is an electrochemical process in which a
systems: galvanic and impressed current. Figure 1-2
current leaves a structure at the anode site, passes
shows these two types. Note that both types have
through an electrolyte, and reenters the structure at
anodes (from which current flows into the
the cathode site as figure 1-1 shows. For example,
1-1



 Previous Page
Previous Page
